
Our advanced EPR dosimetry system meets the highest quality assurance standards, complying with FDA 21 CFR Part 11, ISO, EN, and ISO/ASTM regulations.
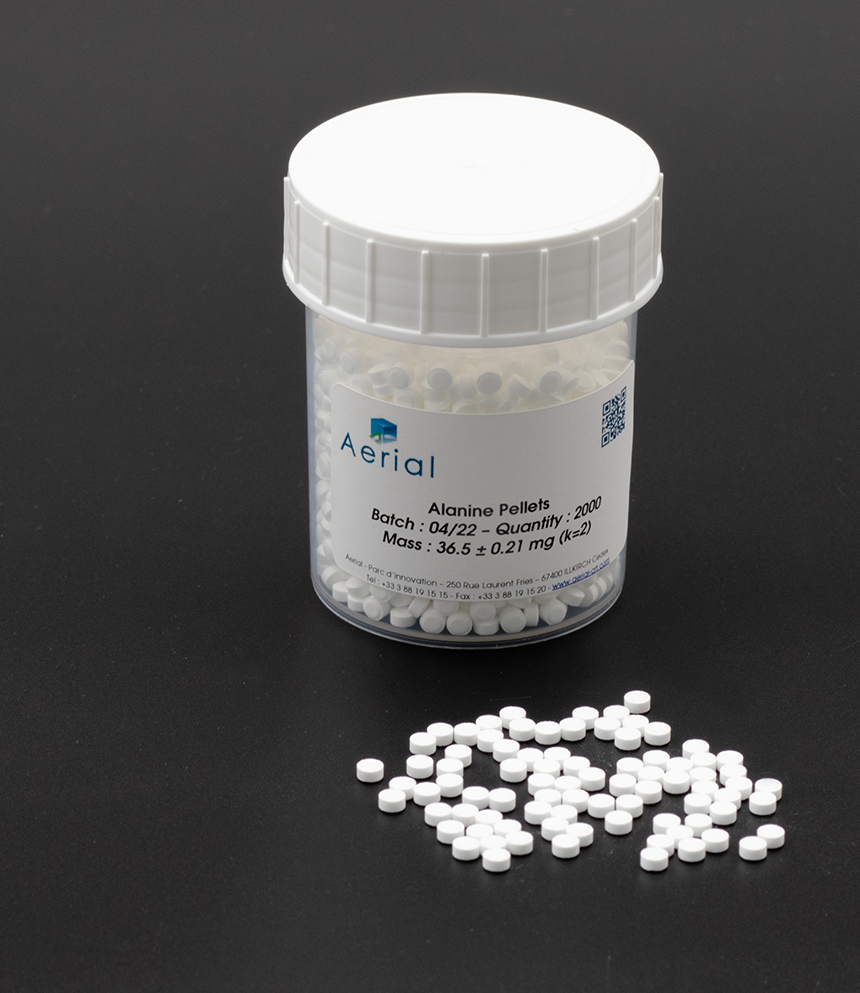
Software is validated and meets all quality assurance requirements according to FDA 21 CFR part 11, ISO, EN, ISO/ASTM… guidance or standards.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.



